Cancer Biology
About
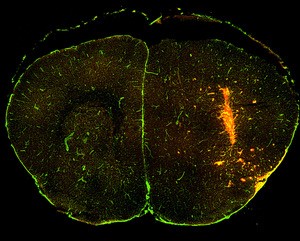
The brain image shows mCherry+ melanoma cells (red) that have dispersed and invaded by migrating along the brain vasculature (green). – Courtesy of Dudley lab
Tumors are living organs, operating to promote the survival of cancerous cells at the expense of the host. Cancer is a complex and systemic process, often arising from dysregulation of gene expression and protein signaling. Changes in the tissue environment also occur, leading to tumor growth, evasion of the immune system, resistance to therapy, and metastatic dissemination to other organs. The Cancer Biology faculty work to understand these problems by investigating the cellular, molecular, and genetic basis of cancer initiation, metastasis, and therapeutic response.
The research in the Cancer Biology arm of the MIC department can be categorized into three broad areas: Understanding cancer at the molecular and cellular level (intracellular); understanding cancer at the organismal level (intercellular); and translational research. Most cancer biology labs have projects encompassing each of these areas.
Some questions that the Cancer Biology faculty are working to understand:
- How does the commensal microbiome impact the function of immune cells in the tissue and tumor microenvironment to facilitate tumor metastasis?
- How do tumors exploit dysfunctional blood vessel to promote cancer progression, therapy resistance, and immune evasion?
- How do cancer associated fibroblasts and endothelial cells control the infiltration, organization, and function of immune cells in tumors?
- How do changes in organelle dynamics impact the biological processes that drive tumor growth?
- How do microenvironmental interactions with pre-neoplastic lesions change during tumor growth?
- What are the molecular events that drive brain tumor formation and progression and how can we exploit them to develop and test new experimental therapies?
- How do dynamic interactions between tumor and normal cells (wild-type sibling, tumor microenvironment, and immune cells) enable robust tumor progression?
- What are the critical nodes in the cell signaling network that contribute to cancer progression and therapeutic resistance?
The Cancer Biology program benefits from a vibrant Cancer Center that has recently achieved the prestigious Comprehensive Designation from the National Cancer Institute, which supports Cancer Biology faculty and students through clinical infrastructure, shared resources, seed grants, and education/outreach.
University of Virginia Cancer Center
The University of Virginia Cancer Center (UVACC) is one of the leading cancer research, management and educational institutions in the USA. UVACC is a matrix cancer center that brings together 162 faculty members from 25 Departments in the Schools of Medicine, Nursing, and Engineering, and in the College of Arts and Sciences. The UVACC has a total of $38.91M in overall yearly direct cost funding. Its members are highly productive and collaborative, having published well over one thousand publications in the last five years, 26% of which in journals with an impact factor of 10 or greater. Through faculty recruitment and robust infrastructure development, the UVACC has continued to build on its exceptional basic science foundations. Its mission is to reduce the burden of cancer with skilled, compassionate care for the patients of today and with research and education for the patients of the future in an environment that promotes diversity, equity, and inclusion. The UVACC enables its members to draw on the deep resources of the University of Virginia to identify, analyze, and validate some of the most significant targets for cancer prevention, screening, diagnosis, and treatment, and to speed their translation to application.
All MIC Cancer Biology faculty are members of UVACC and some have active leadership roles in the center. The UVACC Director, Dr. Thomas Loughran has a faculty appointment in MIC. MIC primary faculty Drs. Amy Bouton (interim chair), Roger Abounader and Jay Fox are Associate Directors for Education, Basic Science and Research Infrastructure at UVACC, respectively. MIC primary faculty Dr. Daniel Gioeli is co-leader of the Cancer Biology Program and MIC primary faculty Dr. David Kashatus is co-leader of the GI Translational Research Team. The close association between MIC and UVACC is enormously mutually beneficial to both entities and to cancer research at UVA.
The Cancer Research Training Program (CRTP)
For almost 50 years, the CRTP at the University of Virginia has brought together faculty and trainees with interests in cancer biology to work toward the common goals of (1) developing a greater understanding of the systems and processes that drive tumor initiation, growth, progression, and metastasis; (2) identifying and exploiting novel and potentially tumor-specific therapeutic targets to help develop new cancer treatments; and (3) empowering a new generation of cancer researchers who have an appreciation for the current state of cancer biology, a vision for the extraordinary advances that will be accomplished in their lifetimes, and the tools to pursue research careers that will contribute to building “a 21st century workforce capable of advancing cancer research using a scientifically integrated approach.” Our program incorporates novel research and educational strategies designed to ensure that our trainees become facile with the newest techniques in cancer research and have a broad-based knowledge of cancer genomics/epigenetics; molecular drivers of cancer; tumor immunology; contributions of the tumor microenvironment to tumor control, growth and metastasis; and drug discovery/delivery. Equally important, the CRTP aims to develop trainees who are adept in partnering with basic scientists, translational researchers, clinical trialists, and clinicians to promote discovery and improved outcomes in cancer care. This is the only training grant at the University of Virginia with this focus, thus fulfilling a specific niche and an important need at our institution while capitalizing on an interactive cancer research and clinical care community.
A defining characteristic of this long-standing training program is the commitment on the part of its leadership to keep it fresh and scientifically relevant. This is accomplished through frequent assessments of the cancer research enterprise; input from cancer training faculty outside of the training program as well as within UVA; and input from current and past trainees. Presently, we have developed several new enhancements to the CRTP which includes: (1) a trainee-run monthly workshop; (2) grant-brewing sessions; (3) applied learning activities; (4) patient-trainee partnerships; (5) enhanced training in rigor and reproducibility through a partnership with the Center for Open Science; (6) mentor training; and (7) a new Advisory Committee for Diversity and Inclusion. The first four of these are trainee-centric, reinforcing community-building and providing new opportunities for grant-writing support, professional enrichment, and leadership. The remaining enhancements are designed to recognize the importance of scientific rigor in research, foster strong mentorship, and promote diversity in our learning/training community. The CRTP program leaders are Dr. Amy Bouton and Dr. Andrew Dudley. For more information visit the cancer research training program.
Faculty
Our Cancer Biology faculty have a strong commitment to translational science. Faculty work closely with clinical colleagues and the UVA Cancer Center to translate basic discoveries into clinical advances or to use clinical specimens and human-based models to ask basic questions related to cancer biology, cancer immunology, and the tumor microenvironment.

Engelhard, Victor H.
Identification of MHC-restricted tumor antigens / Control of T cell homing to tumors / Tertiary lymphoid structures and intratumoral immunity

Ewald, Sarah
Innate immunity, chronic disease, host-parasite interactions, Toxoplasma gondii, proteomics

Hammarskjöld, Marie-Louise
Post Transcriptional Gene Regulation and the Molecular Biology of Human Retroviruses

Rekosh, David M.
Human Immunodeficiency Virus Gene Expression; Human Endogenous Viruses; SARS-CoV-2 Protein Trafficking; Post-transcriptional Gene Regulation

Rutkowski, Melanie
Influence of commensal microbes on immune homeostasis, anti-tumor immunity, and metastasis











